Topics
- Ideal Gas Law
- Kinetic Molecular Theory
- Diffusion
- PV Work
- Maxwell-Boltzmann Distribution
Description
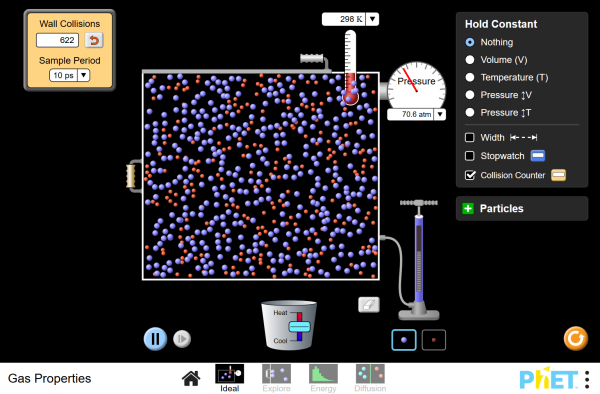
Pump gas molecules to a box and see what happens as you change the volume, add or remove heat, and more. Measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Examine kinetic energy and speed histograms for light and heavy particles. Explore diffusion and determine how concentration, temperature, mass, and radius affect the rate of diffusion.
Sample Learning Goals
- Determine how changing a variable among P, V, N, and T influences other gas properties.
- Describe the relationship between particle-wall collisions and pressure.
- Predict how changing temperature will affect the speed of molecules.
- Predict the speed of molecules in thermal equilibrium based on the relative masses of molecules.
- Identify when pressure-volume work is done on or by a gas.
- Explain how two gases mix.
- Design an experiment to find the factors which affect the rate of diffusion.