Topics
- Beer's law
- Solutions
- Concentration
- Molarity
- Light
- Absorbance
- Transmittance
- Spectrophotometry
Description
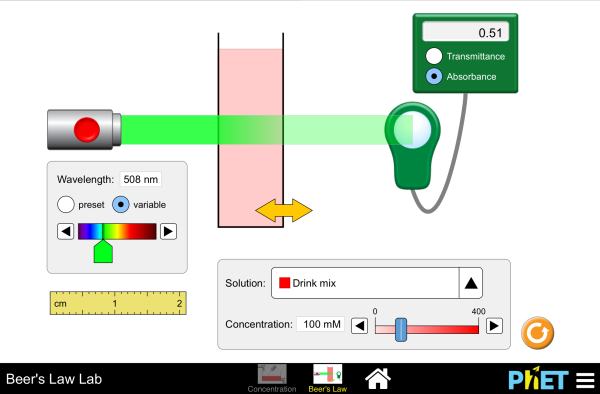
“The thicker the glass, the darker the brew, the less the light that passes through.” Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer!
Sample Learning Goals
- Describe the relationships between volume and amount of solute to solution concentration
- Explain qualitatively the relationship between solution color and concentration
- Predict and explain how solution concentration will change for adding or removing: water, solute, and/or solution
- Calculate the concentration of solutions in units of molarity (mol/L)
- Design a procedure for creating a solution of a given concentration
- Identify when a solution is saturated and predict how concentration will change for adding or removing: water, solute, and/or solution
- Describe the relationship between the solution concentration and the intensity of light that is absorbed/transmitted
- Describe the relationship between absorbance, molar absorptivity, path length, and concentration in Beer’s Law
- Predict how the intensity of light absorbed/transmitted will change with changes in solution type, solution concentration, container width, or light source, and explain why